INDUSTRIAS CUIDADO DE LA SALUD PROCESOS
INDUSTRIAS CUIDADO DE LA SALUD PROCESOS
Laboratorios y Farmacéuticas
Los estándares de producción de la industria farmacéutica son estrictamente apegados a regulaciones que controlan los contenidos activos, las formulaciones, las cantidades producidas como la esterilización de toda parte que toque el producto y finalmente el control del medio ambiente donde se produce.
El diseño y especificación de sistemas de instrumentación, automatización e información asegura el cumplimiento y trazabilidad de estas regulaciones al mismo tiempo la eficiencia en la operación de los procesos.
Agua de calidad
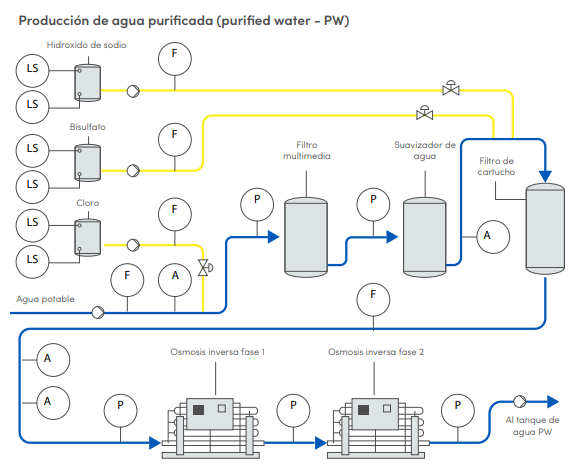
La calidad del agua juega un papel importante en la elaboración de productos farmacéutico como por ejemplo el agua utilizada para soluciones inyectables la cual en su recorrido se deben de monitorear valores analíticos en todo las estas para garantizar la calidad del agua. Otro punto importante es impedir la carga microbiana por lo tanto el agua debe mantenerse en movimiento.
Producción de agua purificada (purified water - PW)
Formulación proceso Batch
La formulación es el paso más exigente en la fabricación de productos farmacéutico y es la clave para determinar la calidad del producto final. La adición y mezcla precisas de materias primas se requiere un control preciso, así como la confiabilidad de instrumentos de medición para garantizar un producto de calidad y que cumpa con las entenderes y normativas establecidas
Ventajas de un control Bach
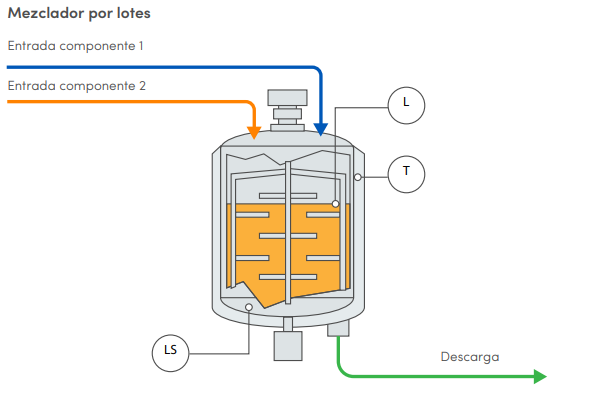
Diagrama de mezclador por lotes
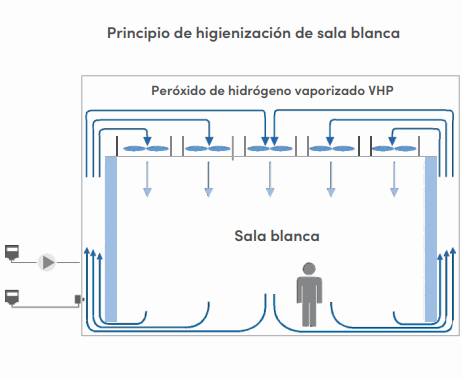
Bio-descontaminación de Salas Blancas
El peróxido de hidrógeno vaporizado (VHP) se genera al vaporizar activamente una solución acuosa de peróxido de hidrógeno e inyectarla en una habitación. Alcanzar una tasa alta de bio-descontaminación de microorganismos requiere establecer una concentración y un tiempo de exposición altos.
Por el tipo de proceso y riesgos hacia el personal es necesario el monitoreo de estas salas.
Beneficios del monitoreo constante en cuartos limpios
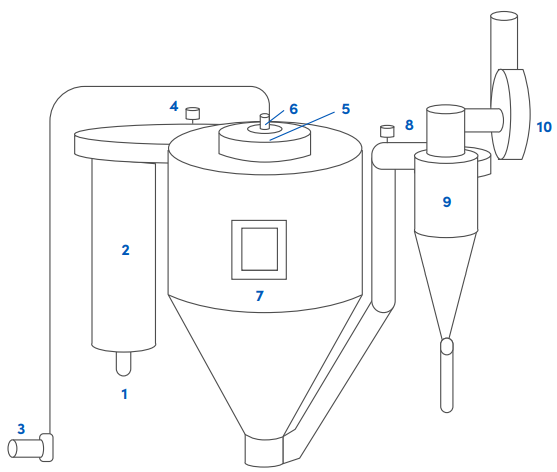
Proceso de granulación
La granulación es una operación que tiene como fin la aglomeración de sustancias finamente divididas o pulverizadas mediante presión o la adición de un aglutinante disperso en un líquido.
Uno de los métodos más utilizados es la granulación húmeda con los sistemas de lecho fluido (fluid-bed systems) o granulación por atomización son sistemas empleados para la desecación y la granulación por vía húmeda.
El proceso de adición de una solución líquida a los polvos implica el amasado de una mezcla de partículas primarias de polvo seco utilizando un fluido de granulación.
Secador atomizador
Componentes principales:
Partes:
Componentes opcionales